as seen in







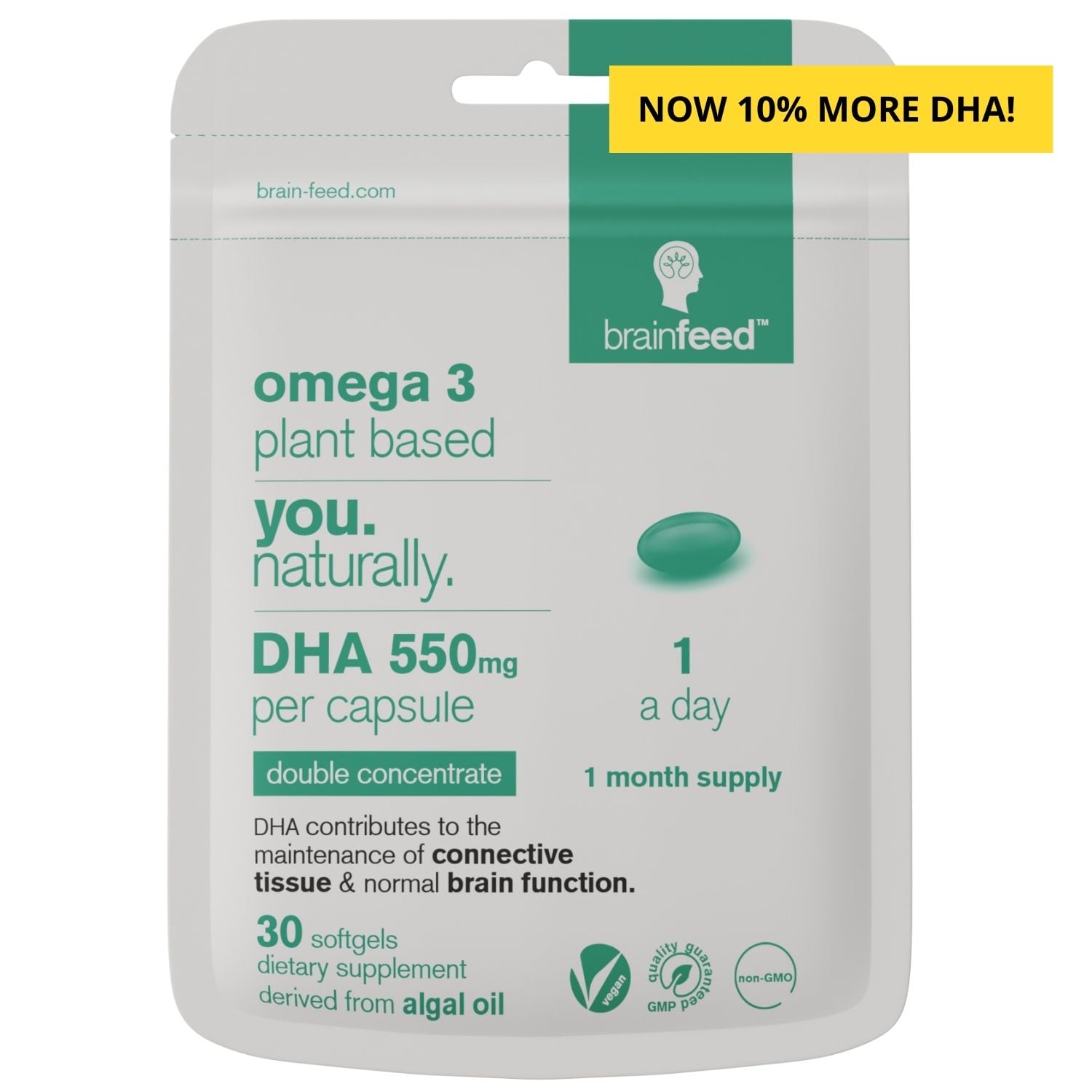
1st double-concentrated DHA supplement derived from algae, containing 550mg DHA in a single plant-based omega-3 capsule. Be confident you’re supporting your long-term brain health as DHA contributes to the maintenance of normal brain function & vision [1]. Maternal DHA intake contributes to the normal brain development of the foetus and breastfed infants [11].
- 1-a-day. Single capsule dose
- 100% more DHA per capsule (compared to normal fish and plant-based omega 3's)
- Ideal for healthy agers, diets low in oily fish and pregnant or breastfeeding mothers
- Free-from marine pollutants and carrageenan
Our omega-3 DHA supplements are manufactured in an FDA-registered facility in the UK under strict GMP, ISO 9001, and ISO 22000 certified conditions.
43 studies link DHA & brain function
brain feed analysed 43 published trials confirming DHA’s role in brain function.


*brain feed does not test or commission studies on animals. This is an analysis of pre-existing studies.
what is DHA ? The omega-3 for brain health
DHA, an omega 3 fatty acid, contributes to the maintenance of normal brain function[1]. Composing 25% of your brains structure [2], it’s DHA rather than omega-3, EPA or any DHA:EPA ratio that has the EU certified brain health claims.
DHA benefits - Invest in your brain's future
Healthy agers
In healthy young adults with diets low in DHA, supplementation improved memory [3]. In 30-54 year olds, omega 3 improved executive function in participants with low DHA levels [4]. As you age, your body's ability to create new connective tissues slows. DHA has neuroprotective effects [5] and supplementation improved working memory [6].
Studies also show that ensuring a high daily omega-3 intake slows down ageing markers, helping the body age more slowly at the biological level compared to its actual age[7,8]


Diets with low intake of oily fish.
68% of US adults and 95% of children are deficient in omega 3, while 75% of British people miss the daily recommended amount of oily fish [9,10].
Pregnant & breastfeeding mothers
Maternal DHA intake contributes to normal brain & eye development of the foetus & breastfed infants [11]. Consumption of omega 3 increases DHA levels in breast milk [12].



how does DHA work ?

DHA supports brain function by helping brain cells adapt and survive as you age, by creating a special compound that helps brain cells grow, connect better, and protect against inflammation [13] .
algae fermentation technology
the evidence


DHA supplements reviewed
| brain feed's omega 3 DHA | Neuromind plus DHA | Nothing fishy omega 3 | Holland and barrett vegan omega 3 | Omvits omega 3 | Vital DHA | |
|---|---|---|---|---|---|---|
| DHA per capsule | 500mg | 300mg | 200mg | 200mg | 150mg | 225mg |
| Serving size | 1-a-day | 1-a-day | 2-a-day | 1-a-day | 2-a-day | 2-a-day |
| Cost per serving | £0.33 | £0.33 | £1.46 | £0.26 | £0.60 | £0.68 |
| High strength | ✅ | ❌ | ❌ | ❌ | ❌ | ❌ |
| Plant-based | ✅ | ❌ | ✅ | ✅ | ✅ | ❌ |
ingredients
purity promise™

Purity has four components:
1. brain feed ensures the high quality of our extracts and isolates through rigorous testing
2. We monitor critical control points during production for quality control
3. We guarantee the amount of active ingredient(s)
4. Preserve the bio-active compounds for the duration of their shelflife.
Click here to read more
FAQ's
What is optimal brain function?
+
-
What is neurogenesis and is it impacted by age?
+
-
Are there other health benefits of DHA?
+
-
Are there any side effects of DHA?
+
-
Could I have a DHA or omega 3 deficiency?
+
-
How do I take it?
+
-
How long does it take to have an effect?
+
-
Does it contain Carrageenan ?
+
-
What does DHA do ?
+
-
Is there a link between low DHA and cognitive decline?
+
-
Does algae oil taste fishy ?
+
-















